Columns
The column was written based on the lecture given at the "TRS Academia Consortium Symposium" held in February 2024.
Background to the Establishment of the First Startup from Kitasato University Faculty of Pharmacy
- The Utilization and Importance of Incubation Support -
I would like to introduce the background of the establishment of PRD Therapeutics (PRD), the first drug discovery venture from Kitasato University Faculty of Pharmacy, and the incubation support that played a crucial role in its founding.
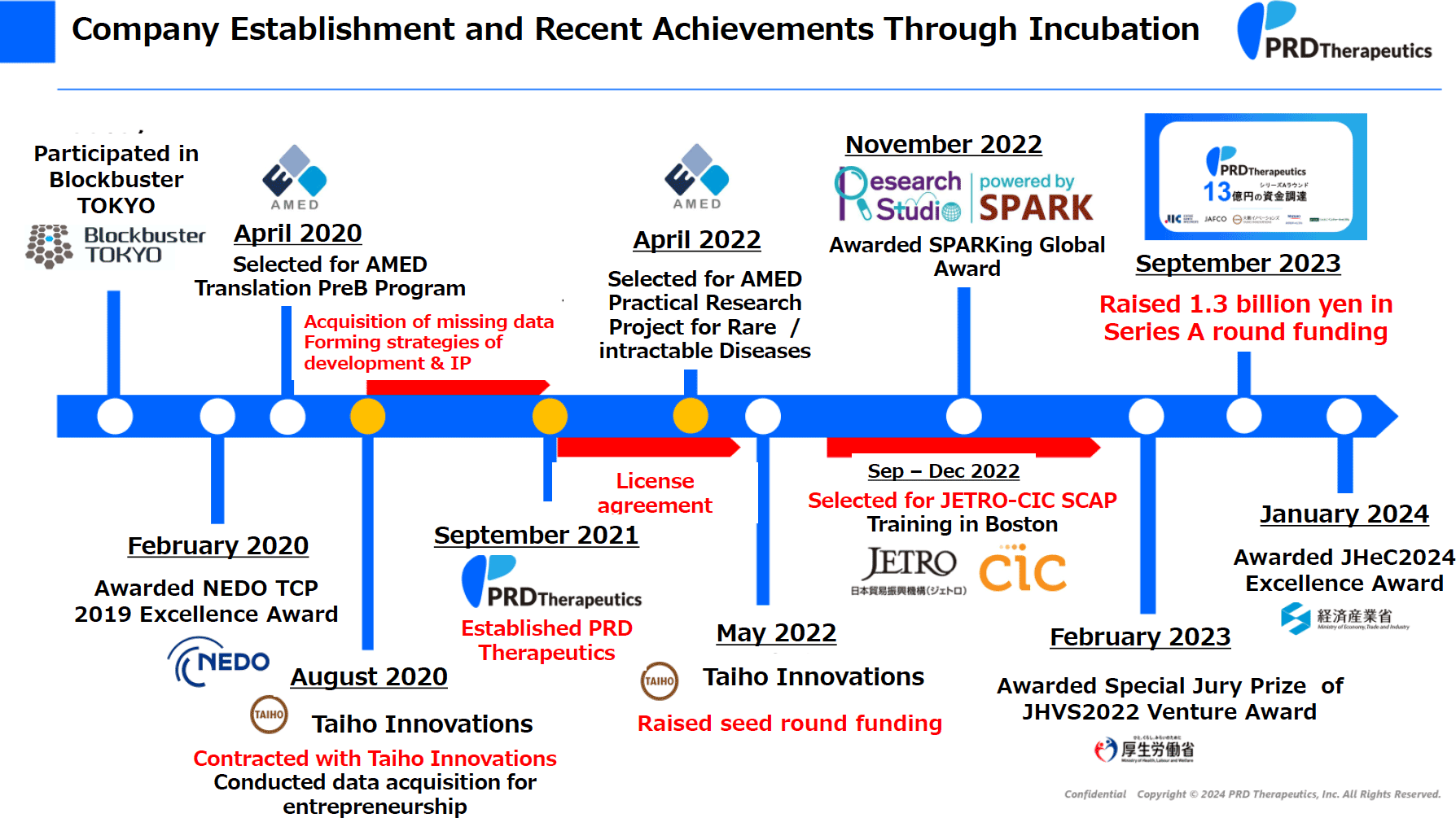
PRD has been developing a compound, PRD001, which was discovered through Kitasato University’s longstanding research on natural products. Kitasato University is well-known for its strength in natural product drug discovery, particularly in microbial metabolites, exemplified by Professor Ōmura’s work on avermectin and ivermectin, which earned him the Nobel Prize in Physiology or Medicine in 2015. My supervisor, Professor Tomoda, also conducted extensive research focusing on the discovery of novel lipid metabolism regulators from natural products under Professor Ōmura. Among these, the most promising compound for drug development was PRD001, an SOAT (ACAT) inhibitor derived from pyripyropene. Coincidentally, the timing of Professor Tomoda’s retirement and the end of my term as a specially appointed assistant professor aligned, prompting us to establish PRD Therapeutics with the goal of commercializing PRD001.
In 2019, at the age of 25, I began considering entrepreneurship and participated in an acceleration program aimed at starting a business. We then entered into an incubation research contract with Taiho Innovations, the corporate venture capital (CVC) arm of Taiho Pharmaceutical, and received one year of incubation support. This support involved acquiring additional data required for commercialization, developing a strategy for drug development, and formulating an intellectual property (IP) strategy. Although negotiating the licensing agreement with the university proved challenging due to the startup’s academic origins, we successfully founded PRD Therapeutics in September 2021. Since then, we have received support from Japan Agency for Medical Research and Development (AMED) for the Practical Research Project for Rare / Intractable Disease and completed a Series A funding round of 1.3 billion yen at the end of 2023.
Initially, our research at the university targeted the large market of chronic diseases. However, considering the scale and duration of clinical trials required, we shifted our focus to a different target disease more suitable for a startup. PRD001 is an excellent lipid metabolism regulator and has demonstrated strong efficacy in animal studies, significantly lowering blood lipid levels, including LDL cholesterol, following oral administration. We believe that PRD001 could be broadly applicable to diseases caused by lipid metabolism disorders. With the support of our incubation program, we conducted research on target diseases, market analysis, and unmet medical needs. Based on these results, we developed a strategy setting the rare and designated intractable disease, homozygous familial hypercholesterolemia (HoFH) as the first target, followed by future expansion to address non-alcoholic steatohepatitis (NASH/MASLD), a liver disease with high unmet medical needs and a large patient population. HoFH is a genetic disorder characterized by elevated blood cholesterol levels from a young age, significantly increasing the risk of cerebral infarction and myocardial infarction in the 30s by progressing arteriosclerosis. In Japan, there are approximately 300 patients, and globally, the number stands at around 30,000, making it a rare disease. While several approved drugs exist, they are often insufficiently effective, requiring patients to undergo extensive treatments, such as LDL apheresis, every one to two weeks for over two hours from infancy, placing a considerable burden not only on the patients but also on their families. We are developing an oral medication, which we believe can be administered at home would significantly reduce this burden and improve the quality of life (QOL) for these patients and their families. A major feature of PRD001 is its mechanism of action. It is the world’s first and only inhibitor that isozyme-selectively targets SOAT2, a key enzyme in lipid metabolism extensively researched at Kitasato University. Approximately 80% of lipids are primarily synthesized in the liver, and the remaining 20% is absorbed from the intestines and enters the bloodstream. SOAT2 plays a crucial role in both processes. In addition, while normally blood lipids are regulated by uptake through LDL receptors, the majority of HoFH patients have genetic mutations in the LDL receptor, preventing proper uptake and leading to excessive lipid accumulation in the blood from a young age. The amount of LDL receptors is regulated by PCSK9, another important target. PRD001 works by inhibiting SOAT2, reducing both hepatic synthesis and intestinal absorption of lipids, thereby lowering blood lipid levels. Additionally, by reducing PCSK9 levels, PRD001 is expected to increase the number of LDL receptors, enhancing the uptake of blood lipids and further lowering lipid levels. The ability to act on all three critical aspects of lipid metabolism, synthesis, absorption, and uptake makes PRD001 a unique and promising compound. So far, it has been demonstrated that SOAT2 inhibition lowers not only LDL cholesterol in the blood but also VLDL and chylomicrons. Additionally, it has become clear that this inhibition reduces not only blood lipids but also liver lipids.
SOAT has attracted the attention of pharmaceutical companies worldwide as a post-statin target, and inhibitor development began around the 1980s. At that time, it was not reported that there were two isoforms of SOAT, which are 1 and 2, and all candidate drugs failed in Phase II or III clinical trials until around 2000. Adverse events observed in the failed clinical trials included increases in blood LDL and worsening atherosclerosis. From the late 1990s to around 2000, it was reported that the existence of two types of SOAT, 1 and 2, and knockout mice for each were created. In SOAT1 knockout mice, worsening atherosclerotic lesions, increased blood lipids, and the presence of xanthomas were observed, reflecting the toxic findings seen in clinical trials. Conversely, SOAT2 knockout mice did not show such toxic effects and demonstrated desired therapeutic effects such as improvements in blood lipids, atherosclerosis, and fatty liver. These findings suggested that selective inhibition of SOAT2 is necessary for developing SOAT inhibitors. All drugs developed by pharmaceutical companies inhibit SOAT1. In this context, the only compound in the world that selectively inhibits SOAT2 is the natural product pyripropen, found at Kitasato University. Although it exhibited strong activity and selectivity, it was found to be metabolically unstable. In collaboration with the synthesis lab of Kitasato University's School of Pharmacy, over 400 derivatives were synthesized from this natural product, conducted lead optimization, and synthesized the compound PRD001, which possesses stronger SOAT2 inhibitory activity, higher selectivity for SOAT2, and improved metabolic stability. All these compounds have been patented by Kitasato University, and PRD, Inc. has obtained exclusive license for their development. Animal tests in mice, rabbits, and monkeys have already been conducted at university, and confirming excellent activity in all cases.
The primary endpoint in clinical trials for HoFH is the reduction rate of LDL cholesterol from baseline, and it has been confirmed that there is a significant decrease from baseline in HoFH model mice. We believe that PRD001 has advantages in terms of efficacy, safety, convenience, and cost. Many existing drugs are considered insufficiently effective because they rely on LDL receptors to lower blood lipids. However, patients with HoFH have mutations in the LDL receptor, resulting in the absence of receptor activity, which means these drugs do not effectively lower blood lipids. On the other hand, animal studies have shown that SOAT2 provides sufficient effects even without active LDL receptors, leading to expectations for its efficacy. Furthermore, while there are many drug candidates being developed overseas, most of them target only PCSK9, which is not effective for patients with HoFH. Moreover, recent modalities such as antibody therapies and gene therapies are often administered by intravenous drip or injection, which, while less impactful on Japan's healthcare system, significantly increases drug costs under the U.S. healthcare system. We believe that the oral administration we are developing offers substantial advantages in terms of convenience and cost. PRD, Inc. is a pipeline-based drug discovery venture focused on PRD001, along with several backup candidates. Currently, PRD001 has completed GLP toxicity testing in preclinical trials, and we are in the process of manufacturing the clinical trial drug. We plan to start Phase I as soon as preparations are complete. Our exit strategy is to seek a partnership or M&A with a pharmaceutical company after obtaining proof of concept (POC) in Phase I. We have developed our clinical trial design and development strategy with the help of incubation support, receiving introductions to experts from the early stages. Our start-up is quite small; while we recently added a new board member in addition to Taiho Innovation Systems which we have been supported since the beginning, the operation has primarily been managed by myself and Dr. Tomita. The ability to run such a small operation has been facilitated by an increase in outsourcing options. We maximize outsourcing for nonclinical trials and CMC while involving advisors where necessary, following a style that is less common in Japan but mainstream in the U.S. ventures. Investors often emphasize the importance of team of human resources when considering investments. However, during the startup phase, we face challenges due to a lack of funding and uncertainty about the necessary human resources, making it unrealistic to offer competitive compensation comparable to pharmaceutical companies. These challenges highlight the issues within Japan's startup ecosystem and the importance of incubation support, based on my experiences in launching PRD, Inc.
Despite the promising data from animal studies at Kitasato University, PRD compounds had not attracted interest from pharmaceutical companies. Now we can understand that this is due to the lack of safety and pharmacokinetic data, however, at that time, we decided to start a start-up, gather more data and eventually sell the compound to a pharmaceutical company. To bridge the gap between research and business, I participated in two programs: Blockbuster TOKYO, sponsored by the Tokyo Metropolitan Government, and Research Studio at University of Tsukuba. These programs provided the necessary knowledge on capital policy, target product profile (TPP), regulatory aspects of drug development, and pitch presentation materials based on what I learnt through the programs, and regulations on drug development. Through the acceleration programs, I got the opportunity to pitch overseas. This pitch in San Francisco led to the initial contact with Mr. Mori from Taiho Innovations, marking the beginning of our incubation journey. The incubation contract between Taiho Innovations and Kitasato University, established before the company’s foundation, set high goals to determine whether PRD compounds were suitable for commercialization by pharmaceutical companies. We worked together to clear each goal, and once we cleared all the goals, we started a business together. We obtained safety and pharmacokinetic data that was lacked in data obtained at university, strengthened intellectual property, conducted research on market and target diseases, formulated a concrete development strategy including plans for clinical trials, and CMC studies to create compounds. With the help of experts and financial support, we achieved all these goals within a year, leading to the successful establishment of PRD Therapeutics in 2021. During the one-year incubation period, I was able to hone my problem-solving skills by addressing issues arising during research and improved my decision-making abilities, essential for a startup CEO. This experience was invaluable to me. For Taiho Innovations, this project represented the first case of their integration model, which aims to nurture both drug discovery seeds and human resources to startup business.
In Boston, a highly advanced drug discovery ecosystem has developed, where startups based on academic research are frequently acquired by large companies, creating a cycle of success and generating serial entrepreneurs who go on to start new ventures. This high liquidity and flow of talent contribute to the vibrant ecosystem. Although the level of academic research in Japan is very high, it has not yet translated into the creation of biotech startups. I believe incubation support is vital to bridge this gap. One major challenge in Japan is the shortage of successful entrepreneurs in the biotech field. Additionally, while the funding for drug discovery support in Japan is steadily increasing, it is still an order of magnitude lower than in the U.S. In that case, the amount of data that can be generated at universities is limited, and even if grants are obtained, the funds are often depleted after one or two outsourced animal studies, making it difficult to accumulate sufficient data. From an investor’s perspective, the lack of data makes the venture too risky to invest in. Consequently, even if a company is established, the initial investment from venture capital (VC) is often insufficient, leading to financial difficulties and a return to academia.
The traditional model for Japanese startups has been platform technology-based, where ventures generate income through contract research or joint projects from the non-clinical stage. This is due in part to the challenges I mentioned. However, there have been recent examples of successful mergers and acquisitions (M&A) in pipeline-based ventures, offering hope that Japan’s drug discovery ecosystem will diversify in terms of out-licensing. The impact of incubation support is significant, as it enables the setting of goals from a corporate perspective, bridging the gap between companies and academic researchers, and leading to successful entrepreneurship. Having data at a level demanded by companies facilitates investment. For pharmaceutical companies, incubating seeds in their focus areas can lead to future partnerships or M&A opportunities, thereby revitalizing the out-licensing of startups.
The most important outcome of increased entrepreneurship is the growth of experienced individuals within the ecosystem. Even if corporate CVCs need to incubate in the first cycle, once these startups succeed or exit, serial entrepreneurs will emerge and start new startups. These individuals, with incubation experience, can then support the next generation of startups. Recently, more individuals with pharmaceutical company backgrounds are joining startups and VCs, contributing to the growth of incubation and company formation support. AMED’s support is also becoming more substantial each year, and I am optimistic about the continued revitalization of Japan’s startup ecosystem. Although incubation support is a heavy commitment for CVCs and VCs, by actively supporting startups before and after their formation, we can continue to activate the drug discovery startup ecosystem. As the first case received incubation support, I would like to continue to run the ecosystem many times in the future.
Kanji Hosoda
President and CEO
PRD Therapeutics, Inc.