Columns
Support of Clinical Application for Breakthrough Seeds from Academia
Many new medicines are approved every year. Where do the discoveries and inventions leading to medicines come from? The antibiotic, penicillin, is found in Penicillium, and digitalis, which has cardiotonic and antiarrhythmic effects, is found in a plant called “Foxglove”. Professor Satoshi Omura was awarded the Nobel Prize in 2015. Do you remember what kind of achievement he made? He discovered a compound called evermectin that is effective against parasites from the microorganism, actinomycete. Enhancement of the efficacy and safety of evermectin led to the production of ivermectin, which is used to control livestock parasites and is effective against onchocerciasis. Onchocerciasis is prevalent in Africa and Latin America, and administration of ivermectin has saved many people from blindness. Thus, discoveries and improvements in compounds play important roles in drug development.
In recent years, basic research has developed rapidly, and various functions of the body, causes of diseases based on genetic abnormalities, differences between normal cells and cancer cells, etc., have been clarified. Technologies such as gene recombination and cell culture have been developed. Based on these advancements, many drugs have been developed, such as erythropoietin, a cytokine that increases red blood cells, and Rituxan, an antibody that targets CD20 that is present on the surface of malignant lymphoma cells. As a representative example of recognition for molecular targeted therapy, Professor Tasuku Honjo was awarded the Nobel Prize in 2018. Cancer immunotherapy has attracted attention and is being developed as a fourth treatment method for cancer in addition to surgery, radiation therapy, and chemotherapy. Treatments to boost immunity to cancer have been developed, but unfortunately, only a few have shown efficacy. In a study at Kyoto University, Dr. Honjo discovered a molecule called PD-1 that is related to immunity, and found that it functions to suppress immunity. Suppressing the action of PD-1 improves immunity. Nivolumab (Opdivo®) is an antibody drug that selectively binds to PD-1 to suppress the action of PD-1. The effect of nivolumab is clear compared to other immunotherapies, and it has been approved for many cancers. In this way, the number of cases in which discoveries in universities have resulted in pharmaceutical products has been increasing. In other countries, many venture companies have been established based on university-originated research. The business model in foreign countries is that venture companies are funded based on discoveries in universities, and successful venture companies are acquired by mega pharmaceutical companies.
Although development of the compounds and antibodies mentioned so far has been done in academia, one of the characteristics of candidates from academia is that many of them are based on entirely novel concepts, such as gene therapy and cell therapy. The following are some examples of seeds from the Institute of Medical Science, the University of Tokyo (IMSUT). Although cancer is therapy resistant, on extremely rare occasions, a cancer shrinks after infection with a virus. Based on advancements in gene recombination technology and viral genetic research, many cancer therapeutic viruses have been developed, and one product was approved for malignant melanoma in the United States. G47Δ, a multiple gene-modified herpes simplex type 1, has been developed at IMSUT as a cancer therapeutic virus (![]() https://www.ims.u-tokyo.ac.jp/glioma/research/index.html). For glioblastoma, a type of brain tumor, a phase II trial has been conducted, and an application from a company for approval is expected. Gene therapy is also becoming popular. Improvements in vectors that carry genes into the body have been made; hereditary diseases are especially good targets. Gene therapy for Duchenne muscular dystrophy has been developed at IMSUT, and a clinical trial is expected soon. Induced pluripotent stem (iPS) cells, which are attracting attention as regenerative medicine, were developed by Professor Shinya Yamanaka of Kyoto University, and he has also won the Nobel Prize. The cells used in regenerative medicine are not limited to iPS cells, and various other types of cells are used. Mesenchymal cells obtained from the umbilical cord have also been developed at IMSUT as cell therapy. Mesenchymal cells have a variety of effects, such as suppression of inflammation and excessive immune response, and promotion of repair at the site of injury. A clinical trial was conducted for acute graft-versus-host disease, which occurs when the immunity of transplanted cells attacks the patient after hematopoietic stem cell transplantation.
https://www.ims.u-tokyo.ac.jp/glioma/research/index.html). For glioblastoma, a type of brain tumor, a phase II trial has been conducted, and an application from a company for approval is expected. Gene therapy is also becoming popular. Improvements in vectors that carry genes into the body have been made; hereditary diseases are especially good targets. Gene therapy for Duchenne muscular dystrophy has been developed at IMSUT, and a clinical trial is expected soon. Induced pluripotent stem (iPS) cells, which are attracting attention as regenerative medicine, were developed by Professor Shinya Yamanaka of Kyoto University, and he has also won the Nobel Prize. The cells used in regenerative medicine are not limited to iPS cells, and various other types of cells are used. Mesenchymal cells obtained from the umbilical cord have also been developed at IMSUT as cell therapy. Mesenchymal cells have a variety of effects, such as suppression of inflammation and excessive immune response, and promotion of repair at the site of injury. A clinical trial was conducted for acute graft-versus-host disease, which occurs when the immunity of transplanted cells attacks the patient after hematopoietic stem cell transplantation.
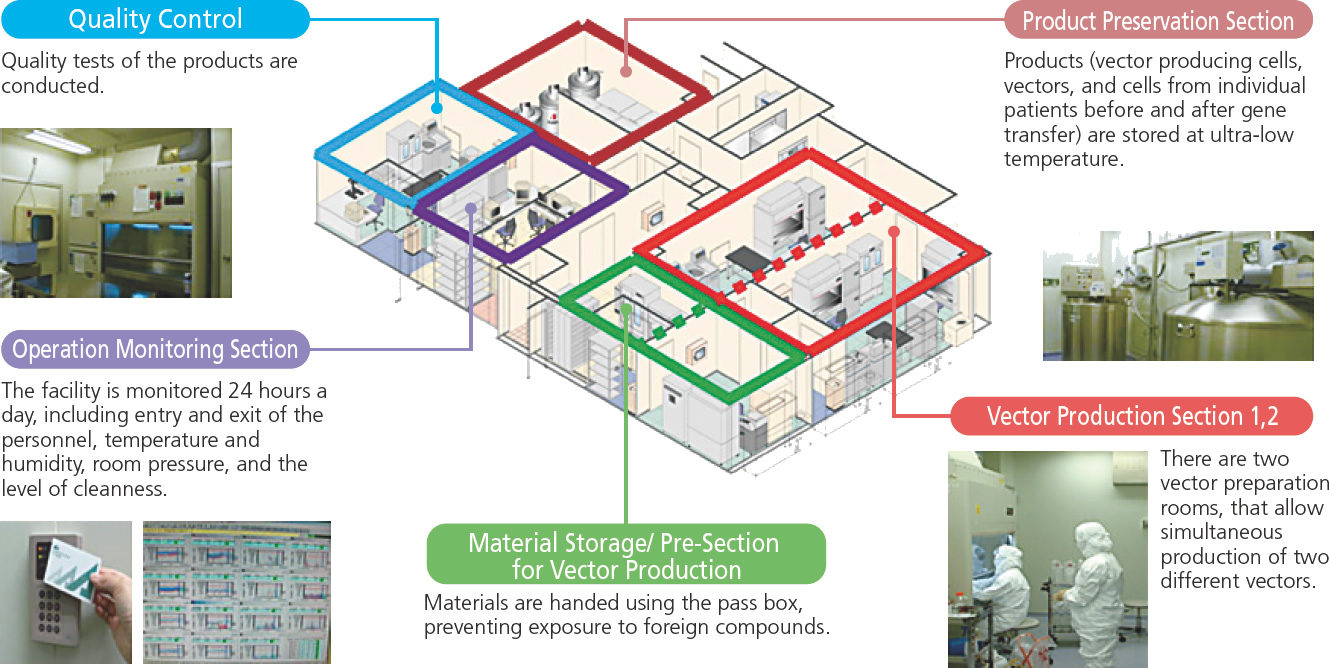
As described above, development in academia is very active. On the other hand, another critical issue is how to obtain gene-recombinant viruses, gene therapy vectors, and cell products for basic research and clinical trials. From the development of seeds to approval, continuous studies of manufacturing methods and a facility that can manufacture a large amount of products with the quality required for human use are required. Researchers should be involved in manufacturing and maintaining quality. IMSUT has established the Therapeutic Vector Development Center (TVDC) for the production of recombinant viruses and vectors for gene therapy. Below is a sketch of the TVDC, which includes two rooms for manufacturing. G47Δ used in clinical trials was manufactured here.

Manufacture of recombinant virus
IMSUT also has a manufacturing room outside the TVDC for preparing gene therapy vectors required for preclinical studies. In addition, the IMSUT Cell Resource Center was established for the production of cellular products, where umbilical cord-derived mesenchymal cells for a clinical trial were manufactured.
Manufacturing of products for clinical trials requires facilities, experienced staff, and standard operating procedures. Equipping such facilities by academia is extremely difficult. This is one of the major barriers in the development of seeds by academia. In addition to these manufacturing facilities, IMSUT has many resources, such as a supercomputer for biotechnology, and many developments based on genomic information have emerged. Our unit supports the development of novel seeds from academia by using all resources of IMSUT. If you are interested, please contact us or the Japan Agency for Medical Research and Development.
November 2020
Frumitaka Nagamura, Professor
Director, Center for Translational Research
Institute of Medical Science
The University of Tokyo